Site Selection and Assessment
investing in research-ready sites
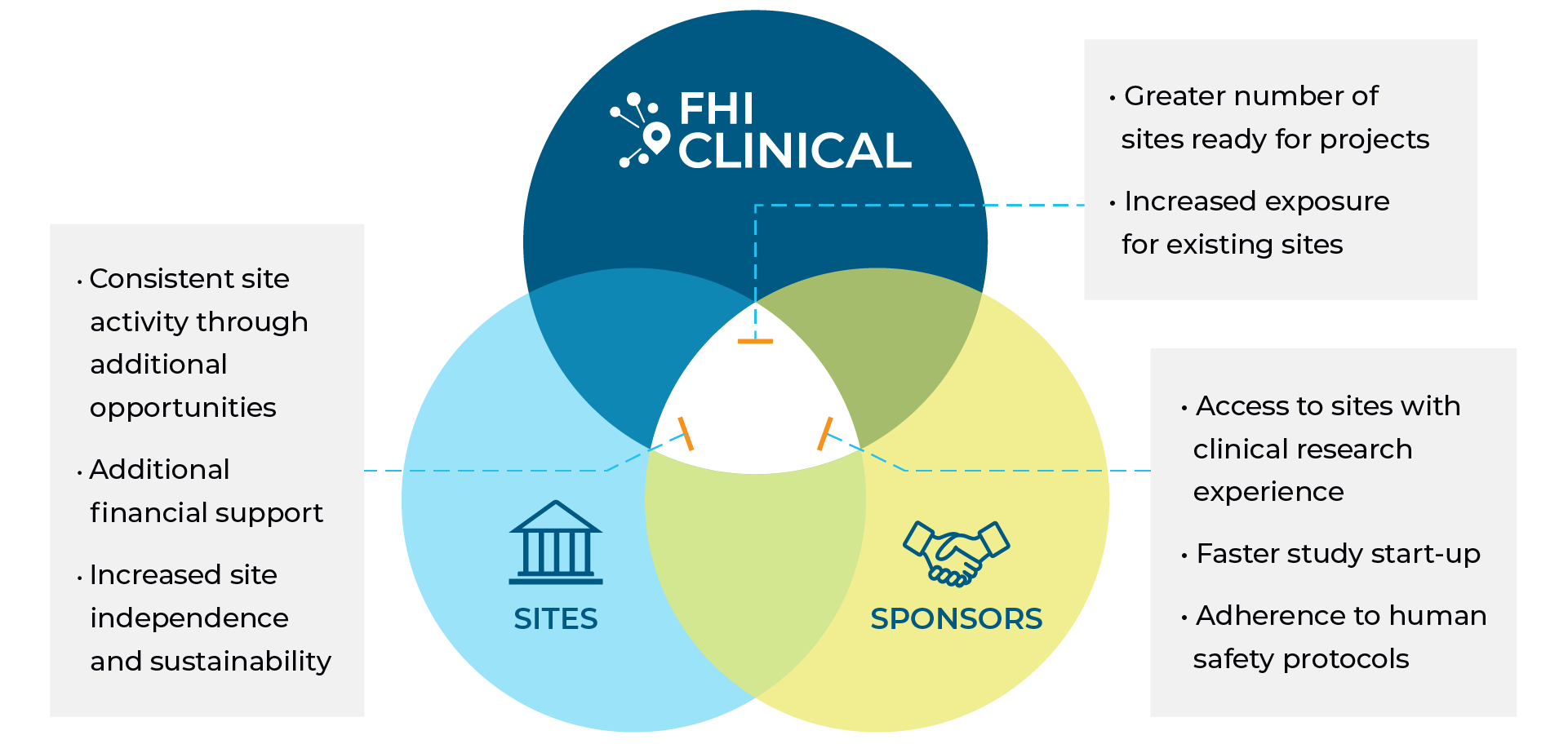
Benefits of Collaboration
scalable, global site selection
Established Relationships
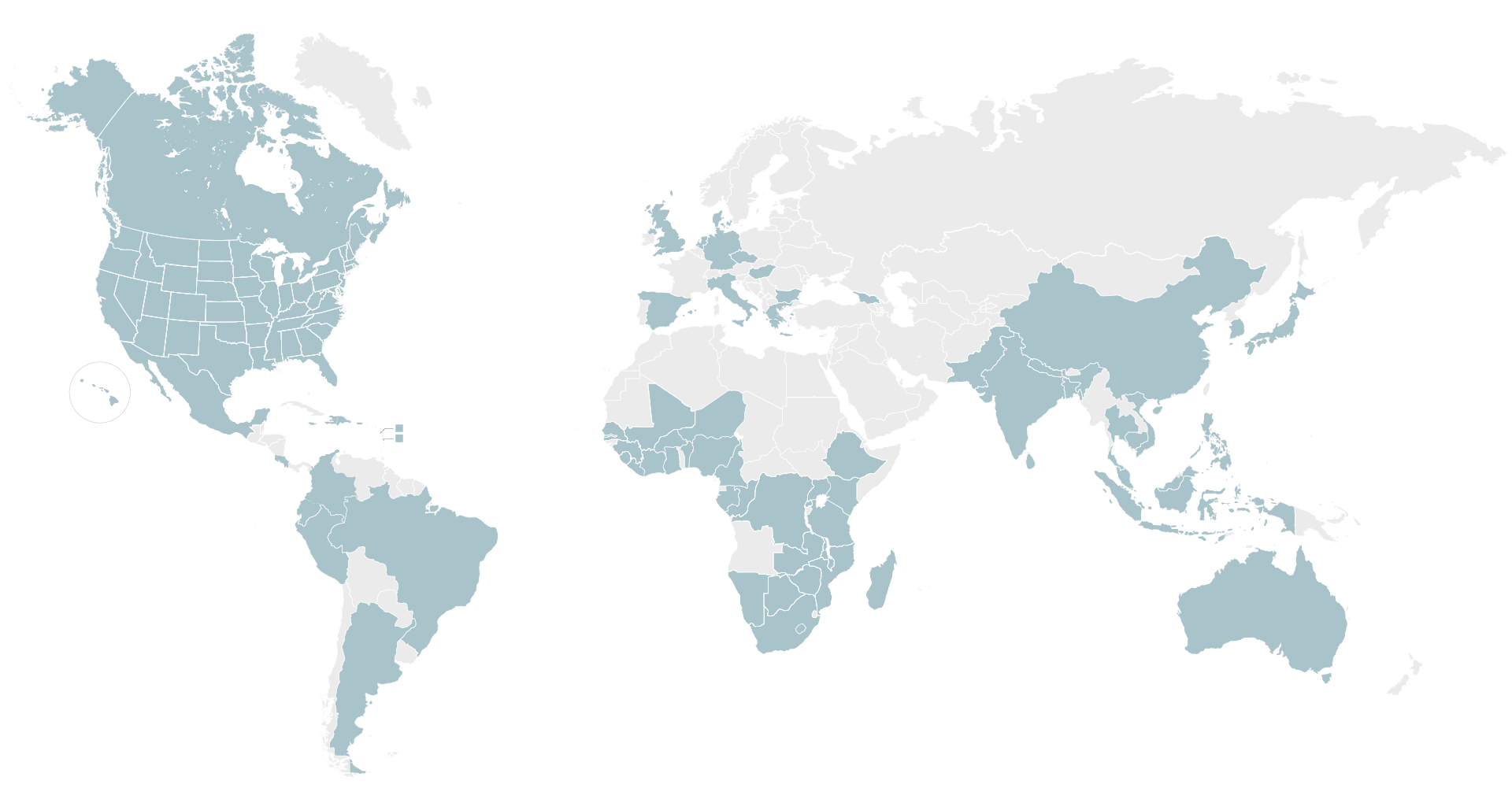
We rely on our established network of ~2,800 sites in nearly 75 countries worldwide that have the required infrastructure and community engagement capabilities to help locate, recruit and retain patients for observational and epidemiological studies and across all clinical trial phases, from Phase 1 to Phase 4.
Would your site like to participate in FHI Clinical's studies?
Provide your site information to be evaluated as a potential partner.
Our Involvement in Research Networks



Top 9 Indications Supported by Our Database of Sites
- Chikungunya
- COVID-19
- Dengue fever
- Diabetes
- HIV
- Hypertension
- Influenza
- Malaria
- Tuberculosis
Of the sites in our database:
Competitive Intelligence and Analytics
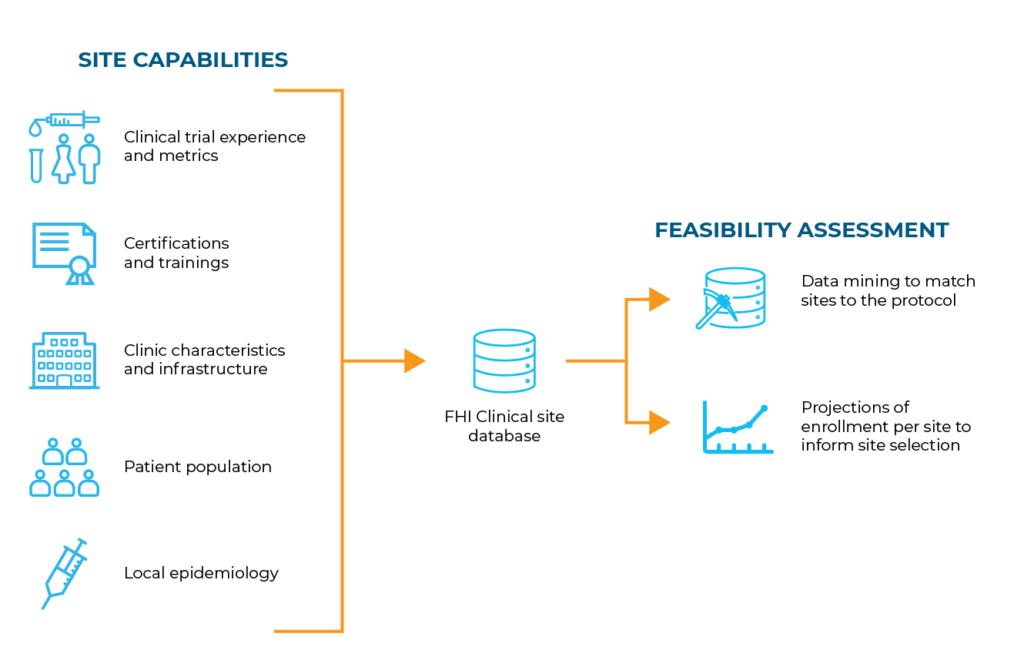
Using our proprietary database of thousands of established, high-quality sites, our Global Strategy team analyzes their capabilities and populations served against your protocol requirements. This feasibility assessment continues throughout the study to ensure successful performance.
comprehensive, customized site assessment
Site feasibility assessments ensure that the “ideal sites” are identified for a specific protocol. To achieve this, we’ve developed assessment strategies and tools based on industry best practices, input from our team of Scientific Advisory Experts and our experience with sites that successfully contribute to studies.
Our site feasibility assessments incorporate the following:
- Interviews with field staff
- Population demographics
- Local disease incidence data
- Survey question design and overall strategy
- Local and national regulatory and ethics processes
- Surrounding political environment
- Number and availability of staff
- Investigator and staff experience in clinical trials and the disease/condition of interest
- GCP training status
- Level of community engagement
- Evaluation of laboratory capacity and experience with the disease/condition of interest
- Specimen collection, processing and storage capabilities
- Pharmacy and pharmacist capabilities
- Facility equipment, maintenance and back-up
- Process of onboarding sites












