Focus On Sites
By working with a range of capable sites, we can reach at-risk populations, bring interventions to those who need it most and address public health issues around the globe.
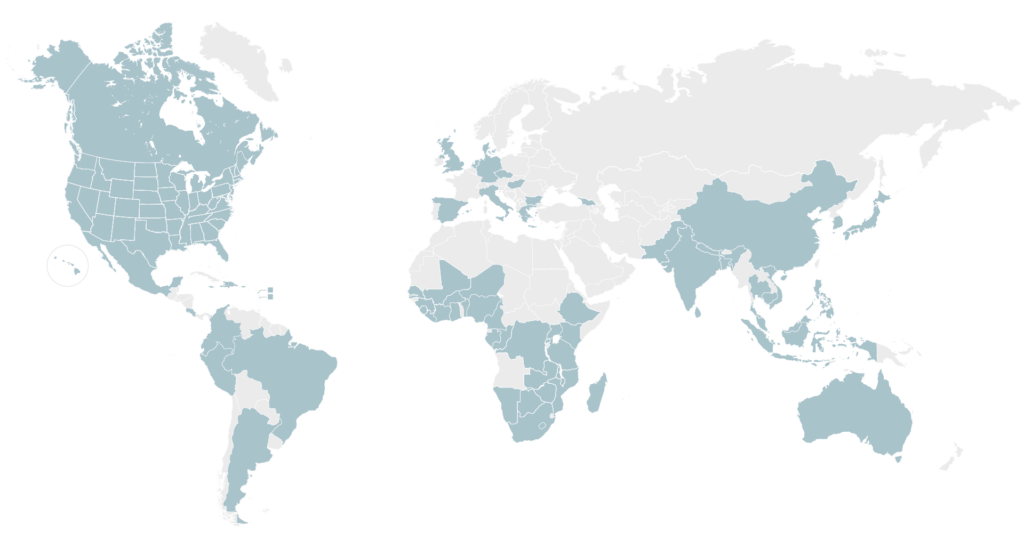
WE HAVE RELATIONSHIPS WITH SITES WORLDWIDE
Our database of research-ready sites includes approximately 2,800 sites worldwide, with experience spanning all clinical trial phases, infectious and non-communicable diseases as well as pediatric and adult populations.
Are you a site interested in being considered for future studies with FHI Clinical?
Complete the form, and we will contact you to arrange a formal introduction with the FHI Clinical team for further evaluation of your site.
WE PARTNER WITH SITES
- The FHI Clinical website and social media
- Meeting FHI Clinical staff in person via a conference or other event
- Networking and referrals to FHI Clinical
- A survey is sent to the institution, to collect details such as (but not limited to) clinical research experience, disease experience and prevalence, phase and population experience, lab capabilities and contact information.
- FHI Clinical uses the survey information to aid with designing feasibility strategies and site lists for future sponsored studies
- Once an institutional profile has been created, FHI Clinical maintains regular contact with these institutions. This ensures that both parties have current and accurate information about each other’s relevant capacity and bandwidth.
- Sites in the database may also provide insight on protocol design and recruitment strategies to ensure both FHI Clinical and the sponsor have accurate real-world information.
SITE AND INVESTIGATOR SPOTLIGHT
We began working with FHI Clinical at the site level before the formation of Flourish and have enjoyed the free flow of collaboration and communication between our companies. One of our early study successes from the FHI Clinical team resulted in Flourish achieving 348% of the original recruitment goal. We attribute that success to our two organizations being aligned in our values, such as working toward reaching underserved communities by building awareness of research opportunities. FHI Clinical has a true commitment to long-term site engagement, and we appreciate the structured, consistent communication plan and find the entire FHI Clinical team to be knowledgeable and responsive to their site partners' needs.
— Lele Simmons, VP, Strategic Partnerships, Flourish Research
About Flourish
- Founded in 2021
- Clinical Trials of Texas (CTT): the platform site for Flourish, followed by acquisitions of other best-in-class sites
- Wholly owned clinical trial sites with access to diverse populations
- Vision: Advancing the health and wellness of society through clinical trials
- Seth J. Baum, MD: cardiology
- Douglas Denham, DO: healthy participants, metabolic diseases and vaccines
- Manish Jain, MD: immunology, vaccines and COVID-19
- Faisal Fakih, MD: sleep and respiratory disorders
- Cherian Verghese, MD: Alzheimer’s disease
- Steven Schmid, PhD: PBMC processing for clinical trials
- Cardiovascular diseases
- Metabolic diseases
- Central nervous system (CNS) diseases and conditions
- Infectious diseases (vaccines)
- Dermatology
- Early phase
- Gastroenterology
- Infectious diseases (treatments; including COVID-19)
- Men’s health
- Musculoskeletal
- Nephrology
- Nutrition
- Pain management
- Pediatrics
- Pulmonology/respiratory
- Radiological imaging
- Rheumatology
- Sleep disorders
- Urology
- Vascular medicine
- Women’s health
- In-house PBMC services at four labs
- San Antonio, TX
- Chicago/Andersonville, IL
- Winter Park, FL
- Miami, FL
- Facilities for next-generation treatments, such as cannabis and psychedelics
- Recruitment and enrollment of diverse populations
- Early-phase studies
- Three early phase locations (FL and TX) with 62 beds total (40 inpatient beds)
- Outpatient Phase 1 trial experience at seven locations
- First-in-human studies
- Single ascending dose (SAD)/multiple ascending dose (MAD) studies
- Insulin challenges
- Glucose challenges
- Glucose/insulin manipulation
- Lumbar punctures
- Infusions
- Certified FibroScan techs
- Bioequivalence
- Cardiac telemetry
- Vaccines
more spotlights
Lucas Tina, MD, MPH; VIBRI and KEMRI
Kenya
Nsengi Ntamabyaliro, MD, MSc; UPC-PV, University of Kinshasa
Democratic Republic of the Congo (DRC)